Navigating product registration in Indonesia is essential for successful market entry and long-term compliance in Indonesia. Whether you’re registering medical devices, cosmetics, food products, or household, understanding BPOM (Badan Pengawas Obat dan Makanan) and Indonesian ministry of health compliance and regulatory requirements can be complex yet invaluable.
This guide offers a step-by-step overview of Indonesia’s product registration process, addressing the types of products that require registration, key steps, and compliance tips to help you avoid common pitfalls.
Product Registration Requirements Overview in Indonesia
To legally sell certain types of products in Indonesia, businesses must obtain approval from BPOM, Indonesia’s National Agency of Drug and Food Control. BPOM ensures that products are safe, effective, and of high quality. For categories such as medical devices, cosmetics, food supplements, food and beverages products or household, compliance with BPOM requirements is mandatory.
Product registration requirements differ by category and often involve safety testing, documentation, and local representation.
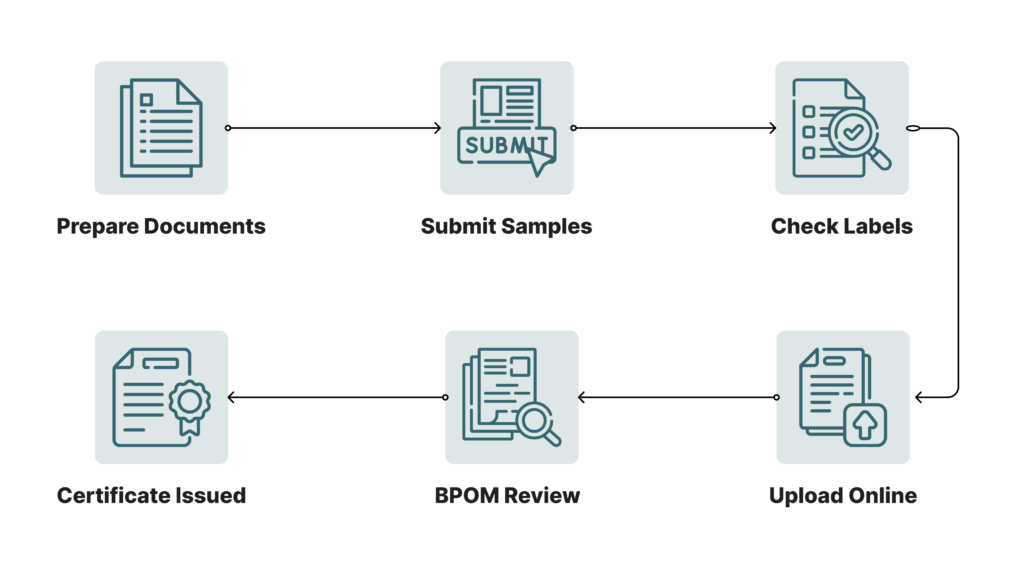
Step-by-Step BPOM Registration Process
Registering a product with BPOM involves several steps, each designed to ensure thorough compliance:
- Preparation of Required Documentation: Each product category has specific documentation requirements. For instance:
· Medical Devices: Ranging from diagnostic tools to therapeutic devices, medical devices must demonstrate safety and effectiveness. BPOM and ministry of health evaluates medical devices to ensure they meet clinical standards, often requiring extensive documentation and quality assurance.
· Cosmetics: Cosmetic products intended for personal care, such as skincare and makeup items, require BPOM registration to ensure they are safe for consumers. This includes ingredient analysis to verify they comply with Indonesian standards.
· Food Supplements: Supplements such as vitamins, minerals, and herbal products must be evaluated for their safety and nutritional content. BPOM ensures that these products contain approved ingredients and meet labeling requirements.
· Food and Beverages: Packaged foods, snacks, and functional beverages all fall under BPOM regulation, which checks for product safety, nutritional labeling, and compliance with import/export standards.
· Household Products: This category includes chemical-based products such as cleaning agents and disinfectants. BPOM assesses these items for potential health risks and proper labeling, ensuring that they are safe for household use.
- Submission of Product Samples: BPOM may request physical samples for laboratory testing, especially for medical devices and certain dietary supplements.
- Review of Product Labels and Packaging: Ensure that all packaging meets BPOM’s requirements for accurate information, warning labels, and language compliance.
- Application Submission via BPOM Online System: BPOM’s online portal allows for streamlined submissions. Upload all required documents, ensuring accuracy and completeness to avoid delays.
- BPOM Review and Approval: BPOM reviews applications thoroughly, and approval times can vary based on the product’s category and complexity.
- Issuance of BPOM Certificate: Upon successful review, BPOM issues a certificate confirming compliance, allowing the product to enter the Indonesian market legally.
Diagram of the BPOM Registration Process
Compliance Requirements for Different Product Categories
Each product category has unique compliance requirements:
- Medical Devices: Must meet standards for safety, efficacy, and quality. This typically includes documentation like the CE mark for imported devices, sterilization information, and clinical trial data if applicable.
- Cosmetics: Compliance with BPOM-approved ingredients and permitted levels of certain substances is crucial. Regular updates to ingredient regulations mean staying informed is key.
- Food and Dietary Supplements: Products need to meet strict nutritional labeling and quality standards. Some require additional testing for allergens or potential contaminants.
- Chemical Products: Requires specific labeling for safety warnings and adherence to proper storage standards, especially for items with potentially hazardous components.
Key Questions Answered
How Long Does the BPOM Registration Process Take?
Depending on the product and category, BPOM registration can take from a few weeks to several months. Factors like application completeness and product complexity significantly impact timelines.
What Documents and Tests are Necessary?
Commonly required documents include certificates of analysis, quality certifications, and product ingredient lists. Testing requirements vary, with food and dietary supplements often needing more thorough allergen testing.
Who Can Assist with Registration?
Given the regulatory intricacies, partnering with a local representative or consultancy specializing in Indonesian product compliance is beneficial. A local agent can ensure your product adheres to BPOM’s specific requirements, saving time and resources.
What Are Common Mistakes in Product Registration?
Frequent errors include incomplete documentation, non-compliance with labeling standards, and misunderstandings regarding BPOM’s specific requirements. Being thorough and enlisting professional assistance can prevent delays and costly rejections.
Benefits of Compliance for Market Success
Compliance with BPOM and Indonesian regulatory standards is critical for legal market entry. Beyond legality, strict adherence ensures consumer trust and long-term market stability. Non-compliance can lead to fines, product recalls, and a damaged reputation, underscoring the value of following regulations closely.
Frequently Asked Questions
- Who Needs BPOM Registration? Businesses that manufacture or import regulated products such as medical devices, cosmetics, food, and chemicals must obtain BPOM certification.
- How Long is the Certification Valid? BPOM certifications typically remain valid for five years, though renewal is necessary to maintain market authorization.
- What are the Costs of BPOM Registration? Costs vary by product category and may include testing fees, document translation, and consultation fees. A local agent can offer a more precise estimate based on your product type.
Final Thoughts
Navigating the BPOM registration process is essential for businesses planning to enter the Indonesian market with regulated products. Thoroughly understanding the requirements, preparing proper documentation, and seeking local expertise are key to a smooth registration journey.
Need help with BPOM registration or understanding Indonesia’s regulatory landscape? Contact us for a free consultation to discuss how we can help streamline your product’s path to compliance and success in Indonesia.




