The recent controversy surrounding a major supplement brand and a proposed class action lawsuit has reignited public attention on the dangers of high-dose vitamin B6 (pyridoxine)—a nutrient often perceived as harmless, yet capable of causing severe neurological harm when consumed excessively.
This isn’t about blaming individuals or companies, but about fostering a deeper, more informed understanding of how supplements interact with our bodies, and the essential role of clear regulatory alignment.
What is Pyridoxine and Why Do We Take So Much?
Vitamin B6 is essential. It’s involved in over 100 enzyme reactions, playing key roles in metabolism, brain development, and immune function. Deficiency is rare in developed countries but can cause anemia and skin issues.
For decades, higher doses of B6 have been popular, often marketed to support energy, reduce symptoms of PMS, or assist in nerve function. The idea that “more is better” or that a water-soluble vitamin can simply be flushed out has been a persistent belief for many consumers.
The Science of Toxicity: Peripheral Neuropathy
Unlike many other B vitamins, B6 is not entirely benign at high levels. The core issue raised in the recent legal action involves peripheral neuropathy, a type of nerve damage.
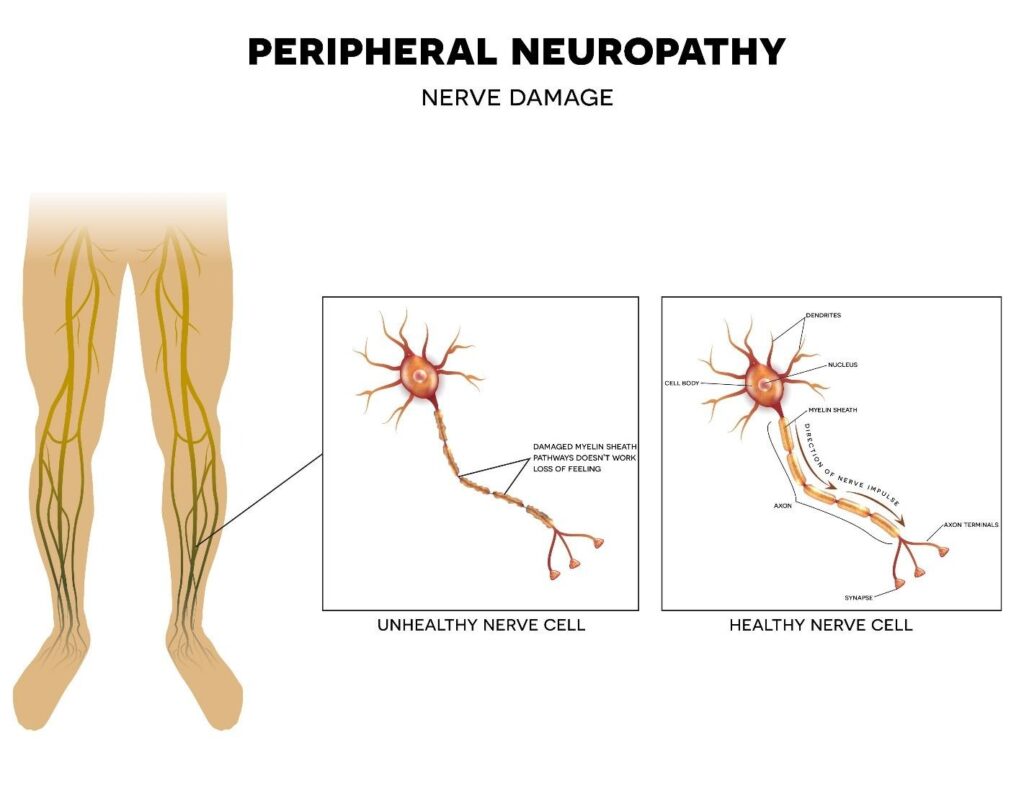
- The Problem: Chronic, high intake of pyridoxine overwhelms the liver’s ability to metabolize it, leading to the accumulation of unmetabolized B6 and its metabolites in the body.
- The Mechanism: These excess compounds are thought to become directly toxic to sensory nerve fibers. Over time, this can result in symptoms ranging from mild tingling and numbness (paresthesia) in the hands and feet to severe nerve pain (neuralgia), muscle weakness, and debilitating coordination issues.
The severity depends on the duration and dose, but the risk significantly increases when the daily intake consistently exceeds the Tolerable Upper Intake Level (UL), which is typically set at 50–100 mg per day for adults.
High-Dose Vitamin B6 in the Regulatory Gray Zone
The key tension here lies in the regulatory framework governing complementary medicines:
- Safety vs. Access: In many markets, the dose legally allowed for sale in a supplement (sometimes 200 mg or higher per day) was set for therapeutic effect, but without adequate controls to prevent chronic user overexposure. This dose often dramatically exceeds the safe, established Tolerable Upper Intake Level (UL).
- Cumulative Burden: A consumer may be taking a multivitamin, a magnesium blend, and a “sleep formula,” all of which contain B6. The cumulative total can easily push the daily intake far into the danger zone, a reality the regulatory system was slow to address.
- The “Up-Scheduling” Response: The reaction to this issue by regulators has been to mandate a change: products containing B6 above a certain daily threshold 50 mg in the case of Australia) are being moved to a “Pharmacist Only” or prescription-based status. This shift acknowledges that these high doses are powerful compounds that require professional oversight, not simply over-the-counter consumables.
A Call for Informed Supplementation
This incident serves as a crucial reminder for all consumers:
- Audit Your Labels: Look closely at the ‘active ingredients’ panel on all your supplements—multivitamins, energy boosters, stress formulas, etc. If the combined daily total of Vitamin B6 (Pyridoxine) exceeds 50 mg, consider discussing it with a healthcare professional.
- Listen to Your Body: Neuropathy symptoms like persistent tingling, numbness, or shooting pain are red flags. If you take a high-dose B6 supplement and experience these symptoms, stop immediately and seek medical advice.
The goal of supplementation is to enhance health, not introduce risk. Issues like vitamin B6 neuropathy highlight the need for stronger:
- Ingredient transparency
- Clearer dosage guidance
- Better alignment between scientific ULs and market regulations
- More robust post-market surveillance
For companies entering tightly regulated markets like Indonesia, ensuring formula safety, dosage compliance, and accurate labeling is not just ethical, but it’s essential for market access.
These cases illustrate that supplement compliance transcends a purely scientific concern; it is, fundamentally, a regulatory strategy. Brands entering Indonesia must ensure that their formulations, claims, and dosage levels align with BPOM standards for dietary supplements and Halal certification requirements.
With Product Registration Indonesia, supplement companies reduce regulatory risk, prevent costly reformulation, and accelerate safe entry into Indonesia’s rapidly growing wellness market.





